The FDA permits AI instruments to foretell breast most cancers danger
Senior Medical Analyst Dr. Mark Siegel discusses the long run dangers of breast most cancers and advances in synthetic intelligence aimed toward predicting a rise in well being dangers from hashish with customers age.
Newou can hearken to Fox News articles!
The US Food and Drug Administration (FDA) has permitted the primary two solely pictures of its variety to forestall HIV, introduced Wednesday by drug creator Gilead Sciences.
The firm’s injectable HIV-1 Capsid inhibitor (Lenacapavir), offered beneath the title Yeztugo, reduces the danger of sexually acquired HIV in adults and adolescents.
Alzheimer’s illness may be prevented by antiviral medication already available on the market
The drug must be administered solely twice a 12 months, nevertheless it exhibits “salient ends in scientific research,” as Gilead argues that it could alter HIV prevention.
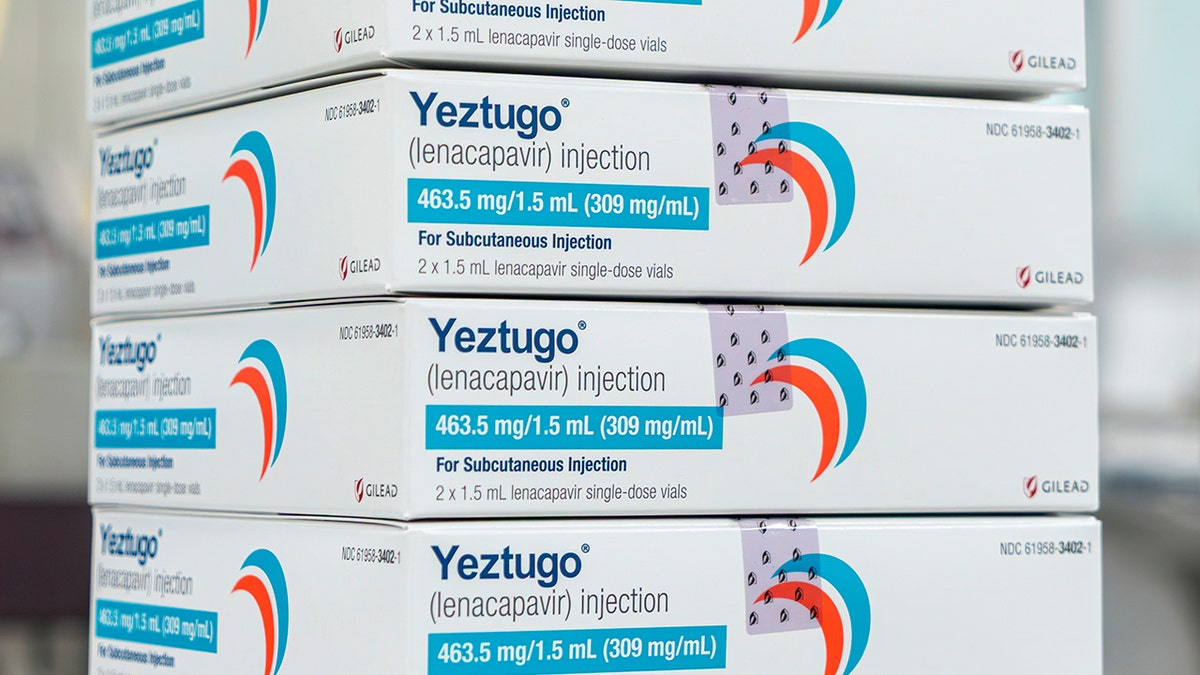
The U.S. Food and Drug Administration permitted Yeztugo, two pictures a 12 months, to forestall HIV, the creator of the drug, introduced Wednesday. (Gilead Sciences by way of AP)
This drug is run as injectable beneath the pores and skin that the physique slowly absorbs. Individuals ought to endure a damaging HIV-1 take a look at earlier than beginning therapy.
Click right here to get the Fox News app
A big trial final 12 months proved that the drug isn’t solely almost 100% efficient in stopping HIV, but additionally superior to a day-to-day oral drug like Truvada, one other drug by Gilead.
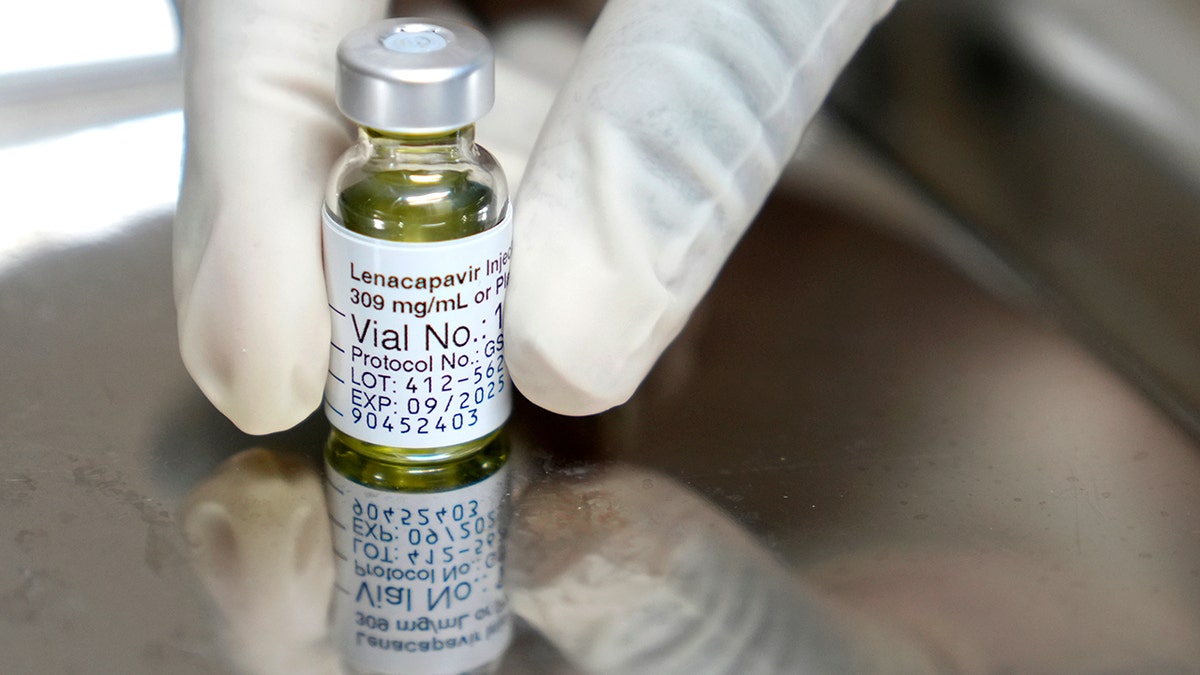
This drug is run as injectable beneath the pores and skin that the physique slowly absorbs. Individuals ought to endure a damaging HIV-1 take a look at earlier than beginning therapy. (AP photograph/nardus engelbrecht)
Journal Science was named the Renacapaville, 2024’s “Breakthrough of the Year.”
Click right here to enroll in our well being e-newsletter
Renacapavir makes use of a multi-stage strategy that distinguishes itself from different permitted antiviral medication.

The firm’s injectable HIV-1 Capsid inhibitor (Lenacapavir), offered beneath the title Yeztugo, reduces the danger of sexually acquired HIV in adults and adolescents. (istock)
For well being articles, please go to www.foxnews.com/well being
The mostly reported unintended effects throughout scientific trials included injection website response, complications and nausea, in response to the corporate.

